Objective. Blood transfusion plays a prominent role in the management of patients with sickle cell disease (SCD) and has a compounded effect on iron overload, especially when simple top-off transfusions are given. Adults with sickle cell anemia (SCA: HbSS or HbSβ 0-thalassemia), often require episodic blood transfusions on a chronic basis and, hence, are at risk of developing transfusional iron overload. We monitored 83 adult SCD patients with a diagnosis of iron overload during routine treatment and follow up at our clinical center. The aim of this study was to observe and describe treatment outcomes for individuals with iron overload seen at the University of Illinois adult sickle cell center between 2010 and 2023.
Method. Patients eligible for the study were aged 18 years or above at the time of their initial visit. Patients were identified from our institutional clinical and translational research informatics data warehouse using computable phenotype algorithms as well as SCD outpatient encounter information to improve accuracy of abstracted data. Results of our queries were abstracted in the form of identified patient data and subsequently reviewed in Epic electronic health record (Epic, Verona, WI). We retrospectively studied adult subjects with SCD seen during routine clinical care between 2010 and 2023. Selected subjects had a diagnosis of iron overload, determined by 2 consecutive ferritin levels >1000ng/ml in steady state plus a significant transfusion history. We extracted data on clinical laboratory markers, abdominal imaging, use of iron chelation at baseline and in the final year of follow up, whether patients were receiving chronic erythrapheresis treatment at baseline and at end of follow up, and if alive at the end of the follow up period. Subjects have variable start date and follow up period, beginning at the start of diagnosis of iron overload and ending in June 2023 or last identifiable follow up visit, or mortality.
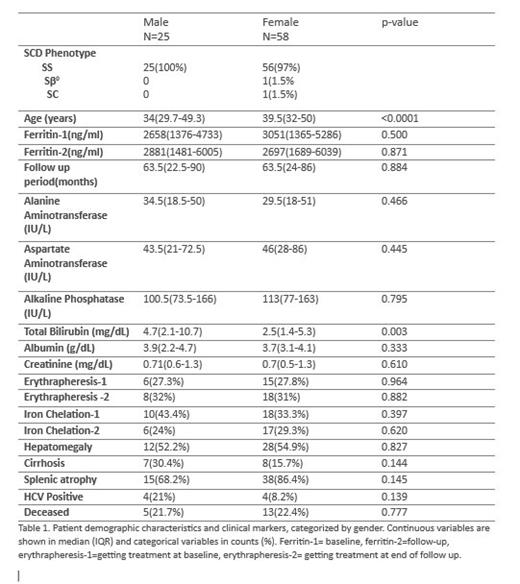
Result. Baseline characteristics of 83 adult patients included in our study are summarized in table 1. Seventy percent of the patients were female and just about everyone had HbSS disease. The females had older median age (39.5year; IQR 32-50 years) than males. The mean ferritin value was significantly higher than the initial measure after a median follows up period of 5.3 years. During the follow up period, 40% of male patients discontinued iron chelation therapy whereas only 5.6% of females did the same. Multivariable analyses were performed for association between change in ferritin measure (between follow up period) with demographic data and other covariates presented in Table 1. Covariates were eliminated by backward selection. Predictors retained in the final model include follow-up period, initial ferritin value, gender, ALT, AST, and albumin. Females had a higher drop in their follow up ferritin, but this was not significant. Neither use of iron chelation therapy at baseline nor erythrapheresis at baseline predicted a change in ferritin value, however we did not collect information on compliance or duration of therapy. Patients followed up for longer duration, or who have higher ALT, had subsequent higher measure for follow up ferritin. Higher albumin predicted a lower ferritin at follow up. The effects of liver markers on ferritin are likely correlative only. After a median follow up period of 5.3years, about 22% of the patients were confirmed to be deceased. Some patients lost to follow up have an indeterminate alive status. Predictors of mortality in multivariate analysis include follow up ferritin value, albumin, and HCV status. Positive HCV status had a strong association with cirrhosis (p=0.001) as 71% of HCV positive individuals had cirrhosis. In turn 53% of the cirrhotic patients died in the follow up period.
Conclusion. Here we present an overview of the clinical outcomes of adult patients with SCD and iron overload during a median follow up period of 5.3 years. Despite limitations of a typical retrospective study, our study illustrates the difficulty associated with the management of transfusional iron overload during routine treatment follow up, especially in patients continuing to undergo episodic transfusion treatment. We also continue to underline the low adherence with chelation therapy in adult populations with SCD and iron overload, and the high mortality associated with the condition.
Disclosures
Saraf:GBT/Pfizer: Consultancy, Other: Advisory board, Research Funding, Speakers Bureau; Forma Therapeutics: Consultancy, Other: Advisory board, Research Funding; Agios: Consultancy, Other: Advisory board; BEAM Therapeutics: Consultancy, Other: Advisory board; Novartis: Consultancy, Other: Advisory board, Research Funding. Gordeuk:CSL-Behring: Consultancy; Emmaus: Consultancy, Research Funding; Forma: Consultancy, Research Funding; Novartis: Research Funding; Modus Therapeutics: Consultancy; Incyte: Research Funding; GBT/Pfizer: Consultancy, Research Funding; Takeda: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal